Tocilizumab won the green light because of its ability to lower aberrant host immune response that leads to an inflammatory cytokine storm -- an overreaction of the immune system -- and death in hospitalised patients.
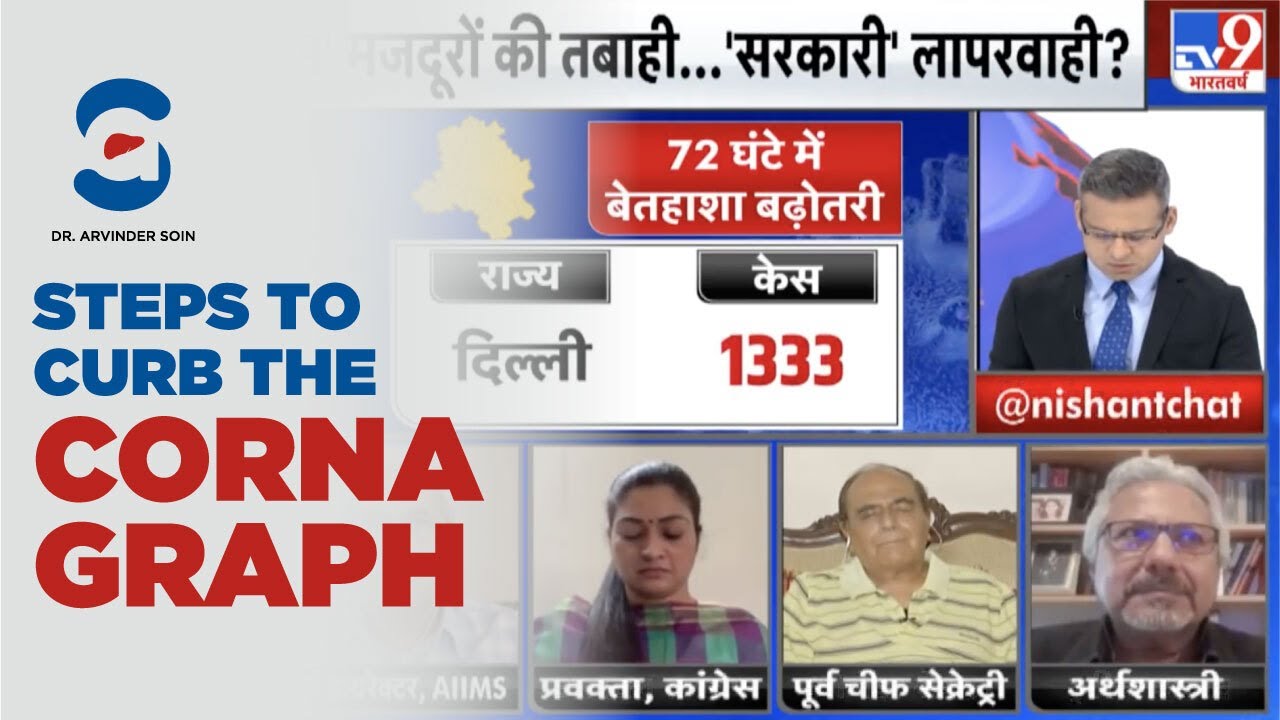
The Central Drugs Standard Control Organisation (CDSCO) on Sunday approved the first multi-centric trial of Tocilizumab in India after the second-line drug to treat rheumatoid arthritis emerged as a treatment option for the coronavirus disease (Covid-19).
Tocilizumab won the green light because of its ability to lower aberrant host immune response that leads to an inflammatory cytokine storm -- an overreaction of the immune system -- and death in hospitalised patients.
Tocilizumab is a monoclonal antibody that acts against interleukin-6 (IL-6), a chemical that causes inflammation. “The deterioration in the Covid-19 patients’ condition in the majority of cases is due to hyper-immunity, or an excessive response of one’s own immune system. This cytokine storm or Cytokine Release Syndrome (CRS) is caused by the release of inflammatory chemicals in the body namely IL-6, TNF-alpha and IL1 among others. Tocilizumab is an antibody against IL-6, which is already available, licenced and effective in treating CRS caused by other conditions,” Dr A S Soin, chairman of the Medanta Liver Transplant Institute, Medanta-The Medicity, Gurugram.
Dr Soin is the national lead investigator for the randomised control trial for the use of Tocilizumab in Covid-19 in India. The trial will be conducted in several hospitals in Delhi-National Capital Region, Haryana, Maharashtra, Uttar Pradesh, Telangana and Tamil Nadu.
For more info click here
Used to treat rheumatoid arthritis, #Tocilizumab got the green light because of its ability to lower the inflammatory overreaction of the human immune system, which leads to death in infected patients.
Earlier this week, India began multi-centre clinical trials of the drug – Tocilizumab for treating COVID-19 following approval from the Central Drugs Standard Control Organisation (CDSCO).
India’s 1st multi-centre trial for COVID19 treatment using Tocilizumab. Many thanks to @drharshvardhan @ICMRDELHI for unparalleled scientific support & accelerated approval.
Wish us luck as we dive in to bring back answers for India & the world!
What is Tocilizumab? As per recent media reports, it is a second-line drug used to treat moderate to severely active rheumatoid arthritis in adult patients.
Once the COVID-19 virus enters the human body, it infects the lung cells, rapidly multiplies, breaks through these cells and infects other cells.
“In this process, the virus has a unique ability to incite a hyper-response of the immune system. It does so by stimulating the release of certain chemicals called cytokines (mainly IL6), which stimulate inflammation called cytokine storm or cytokine release syndrome (CRS), which in turn causes more tissue damage,” states the note issued by Dr Arvinder Singh Soin, chairman of the Medanta Liver Transplant Institute, Medanta-The Medicity, Gurugram and the National Lead Investigator of the trial, to The Better India.
For more info click here